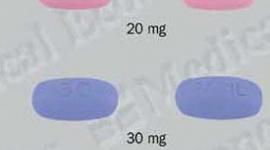
Pristiq (desvenlafaxine) Patient Information
Find out why Pristiq is prescribed, side effects of Pristiq, Pristiq warnings, discontinuation symptoms of Pristiq, more - in plain English.
FDA-Approved Medication Guide and Patient Counseling Information
Pristiq (desvenlafaxine) Full Prescribing Information
Pristiq Medication Guide
PristiqTM (pris-TEEK) Extended-Release Tablets (desvenlafaxine)
Read the Medication Guide that comes with you or your family member's antidepressant medicine. This Medication Guide is only about the risk of suicidal thoughts and actions with antidepressant medicines. Talk to your, or your family member's, healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines
- all treatment choices for depression or other serious mental illness
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
1. Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
2. Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
3. How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
| thoughts about suicide or dying | trouble sleeping (insomnia) |
| attempts to commit suicide | new or worse irritability |
| new or worse depression | acting aggressive, being angry, or violent |
| new or worse anxiety | acting on dangerous impulses |
| feeling very agitated or restless | an extreme increase in activity and talking (mania) |
| panic attacks | other unusual changes in behavior or mood |
What else do I need to know about antidepressant medicines?
- Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms
- Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
- Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child's healthcare provider for more information.
This Medication Guide has been approved by the U.S. Food and Drug Administration for all antidepressants.
Important Information about Pristiq
Read the patient information that comes with Pristiq before you take Pristiq and each time you refill your prescription. There may be new information. If you have questions, ask your healthcare provider. This information does not take the place of talking with your healthcare provider about your medical condition or treatment.
What is Pristiq?
- Pristiq is a prescription medicine used to treat depression. Pristiq belongs to a class of medicines known as SNRIs (or serotonin-norepinephrine reuptake inhibitors).
- Pristiq has not been studied orapproved for use in children and adolescents.
Who should not take Pristiq?
Do not take Pristiq if you:
- are allergic to desvenlafaxine, venlafaxine or any of the ingredients in Pristiq. See the end of this Medication Guide for a complete list of ingredients in Pristiq.
- currently take or have taken within the last 14 days, any medicine known as an MAOI. Taking an MAOI with certain other medicines, including Pristiq, can cause serious or even life-threatening side effects. Also, you must wait at least 7 days after you stop taking Pristiq before you take any MAOI.
What should I tell my healthcare provider before taking Pristiq?
Tell your healthcare provider about all your medical conditions, including if you:
- have high blood pressure
- have heart problems
- have high cholesterol or high triglycerides 34
- have a history of a stroke
- have glaucoma
- have kidney problems
- have liver problems
- have or had bleeding problems
- have or had seizures or convulsions
- have mania or bipolar disorder
- have low sodium levels in your blood
- are pregnant or plan to become pregnant. It is not known if Pristiq will harm your unbor baby.
- are breastfeeding. Pristiq can pass into your breast milk and may harm your baby. Talk with your healthcare provider about the best way to feed your baby if you take Pristiq.
Serotonin syndrome
A rare but potentially life-threatening condition called serotonin syndrome can happen when medicines such as Pristiq are taken with certain other medicines. Serotonin syndrome can cause serious changes in how your brain, muscles and digestive system work. Especially tell your healthcare provider if you take the following:
- medicines to treat migraine headaches known as triptans
- medicines used to treat mood disorders, including tricyclics, lithium, selective serotonin reuptake inhibitors (SSRIs), or serotonin norepinephrine reuptake inhibitors (SNRIs)
- silbutramine
- tramadol
- St. John's Wort
- MAOIs (including linezolid, an antibiotic)
- tryptophan supplements
Ask your healthcare provider if you are not sure if you are taking any of these medicines.
Before you take Pristiq with any of these medicines, talk to your healthcare provider about serotonin syndrome. See " What are the possible side effects of Pristiq?"
Pristiq contains the medicine desvenlafaxine. Do not take Pristiq with other medicines containing venlafaxine or desvenlafaxine.
How should I take Pristiq?
- Take Pristiq exactly as your healthcare provider has told you.
- Take Pristiq at about the same time each day.
- Pristiq may be taken either with or without food.
- Swallow Pristiq tablets whole with fluid. Do not crush, cut, chew, or dissolve Pristiq tablets because the tablets are time released.
- When you take Pristiq, you may see something in your stool that looks like a tablet. This is the empty shell from the tablet after the medicine has been absorbed by your body.
- It is common for antidepressant medicines such as Pristiq to take several weeks before you start to feel better. Do not stop taking Pristiq if you do not feel results right away.
- Do not stop taking or change the dose of Pristiq without talking with your healthcare provider, even if you feel better.
- Talk with your healthcare provider about how long you should use Pristiq. Take Pristiq for as long as your healthcare provider tells you to.
- If you miss a dose of Pristiq, take it as soon as you remember. If it is almost time for your next dose, skip the missed dose. Do not try to "make up" for the missed dose by taking two doses at the same time.
- Do not take more Pristiq than prescribed by your healthcare provider. If you take more Pristiq than the amount prescribed, contact your healthcare provider right away.
- In case of an overdose of Pristiq, call your healthcare provider or poison control center, or go to the emergency room right away.
What should I avoid while taking Pristiq?
- Do not drive a car or operate machinery until you know how Pristiq affects you.
- Avoid drinking alcohol while taking Pristiq.
What are the possible side effects of Pristiq?
Pristiq can cause serious side effects including:
- See the beginning of this Medication Guide - Antidepressant Medicines, Depression and other Serious Mental Illnesses, and Suicidal Thoughts or Actions.
- Serotonin syndrome. See "What should I tell my healthcare provider before taking Pristiq?"
Get medical help right away if you think that you have serotonin syndrome. Signs and symptoms of serotonin syndrome may include one or more of the following:
| restlessness | increase in blood pressure |
| hallucinations (seeing and hearing things that are not real) | diarrhea |
| loss of coordination | coma |
| fast heart beat | nausea |
| increased body temperature | vomiting |
- Pristiq may also cause other serious side effects including:
- New or worsened high blood pressure (hypertension). Your healthcare provider should monitor your blood pressure before and while you are taking Pristiq. If you have high blood pressure, it should be controlled before you start taking Pristiq.
- Abnormal bleeding or bruising. Pristiq and other SNRIs/ SSRIs may cause you to have an increased chance of bleeding. Taking aspirin, NSAIDs (non-steroidal anti-inflammatory drugs), or blood thinners may add to this risk. Tell your healthcare provider right away about any unusual bleeding or bruising.
- Glaucoma (increased eye pressure)
- Increased cholesterol and triglyceride levels in your blood
- Symptoms when stopping Pristiq (discontinuation symptoms). Side effects may occur when stopping Pristiq (discontinuation symptoms), especially when therapy is stopped suddenly. Your healthcare provider may want to decrease your dose slowly to help avoid side effects. Some of these side effects may include:
- dizziness
- nausea
- headache
- irritability
- sleeping problems
- anxiety
- abnormal dreams
- tiredness
- sweating
- diarrhea
- Seizures (convulsions)
- Low sodium levels in your blood. Symptoms of this may include: headache, difficulty concentrating, memory changes, confusion, weakness and unsteadiness on your feet. In severe or more sudden cases, symptoms can include: hallucinations (seeing or hearing things that are not real), fainting, seizures and coma. If not treated, severe low sodium levels could be fatal.
Contact your healthcare provider if you think you have any of these side effects.
Common side effects with Pristiq include:
- nausea
- tiredness
- headache
- diarrhea
- dry mouth
- vomiting
- sweating
- anxiety
- dizziness
- tremor
- insomnia
- dilated pupils
- constipation
- decreased sex drive
- loss of appetite
- delayed orgasm and ejaculation
- sleepiness
These are not all the possible side effects of Pristiq. Tell your healthcare provider about any side effect that bothers you or does not go away. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. For more information on these and other side effects associated with Pristiq, talk to your healthcare provider visit our web site at www.pristiq.com or call our toll-free number 1-888-Pristiq.
How should I store Pristiq?
- Store Pristiq at 68° to 77°F (20° to 25°C)
- Do not use Pristiq after the expiration date (EXP), which is on the container. The expiration date refers to the last day of that month.
- Keep Pristiq and all medicines out of the reach of children.
General Information about the safe and effective use of Pristiq
Medicines are sometimes used for conditions that are not mentioned in Medication Guides. Do not use Pristiq for a condition for which it was not prescribed. Do not give Pristiq to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about Pristiq. If you would like more information, talk with your healthcareprovider. You can ask you pharmacist or healthcare provider for information about Pristiq that is written for healthcare professionals. For more information, go to www.pristiq.com or call 1-888-Pristiq (774-7847).
What are the ingredients in Pristiq?
Active ingredient: desvenlafaxine
Inactive ingredients: hypromellose, microcrystalline cellulose, talc, magnesium stearate, a film coating which consists of sodium carboxymethylcellulose, maltodextrin, dextrose, titanium dioxide, stearic acid and iron oxide(s).
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Issued February 2008
Contact Information
Please visit our web site at www.pristiq.com, or call our toll-free number 1-888-Pristiq to receive more information.
This product's label may have been updated. For current package insert and further product information, please visit www.wyeth.com or call our medical communications department toll-free at 1-800-934-5556.
Wyeth®
Wyeth Pharmaceuticals Inc.
Philadelphia, PA 19101
W10529C002
ET01
Rev 04/08
Patient Counseling Information
Advise patients, their families, and their caregivers about the benefits and risks associated with treatment with Pristiq and counsel them in its appropriate use.
Advise patients, their families, and their caregivers to read the Medication Guide and assist them in understanding its contents. The complete text of the Medication Guide is reprinted at the end of this document.
Suicide Risk
Advise patients, their families and caregivers to look for the emergence of suicidality, especially early during treatment and when the dose is adjusted up or down [see Box Warning and Warnings and Precautions (5.1)].
Concomitant Medication
Advise patients taking Pristiq not to use concomitantly other products containing desvenlafaxine or venlafaxine. Healthcare professionals should instruct patients not to take Pristiq with an MAOI or within 14 days of stopping an MAOI and to allow 7 days after stopping Pristiq before starting an MAOI [see Contraindications ( 4.2)].
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome, particularly with the concomitant use of Pristiq and triptans, tramadol, tryptophan supplements or other serotonergic agents [see Warnings and Precautions ( 5.2) and Drug Interactions ( 7.3)].
Elevated Blood Pressure
Advise patients that they should have regular monitoring of blood pressure when taking Pristiq [see Warnings and Precautions ( 5.3)].
Abnormal Bleeding
Patients should be cautioned about the concomitant use of Pristiq and NSAIDs, aspirin, warfarin, or other drugs that affect coagulation since combined use of psychotropic drugs that interfere with serotonin reuptake and these agents has been associated with an increased risk of bleeding. [see Warnings and Precautions ( 5.4)].
Narrow-angle Glaucoma
Advise patients with raised intraocular pressure or those at risk of acute narrow-angle glaucoma (angle-closure glaucoma) that mydriasis has been reported and they should be monitored [see Warnings and Precautions ( 5.5)].
Activation of Mania/Hypomania
Advise patients, their families and caregivers to observe for signs of activation of mania/hypomania [see Warnings and Precautions ( 5.6)].
Cardiovascular/Cerebrovascular Disease
Caution is advised in administering Pristiq to patients with cardiovascular, cerebrovascular, or lipid metabolism disorders [see Adverse Reactions ( 6.1) and Warnings and Precautions ( 5.7)].
Serum Cholesterol and Triglyceride Elevation
Advise patients that elevations in total cholesterol, LDL and triglycerides may occur and that measurement of serum lipids may be considered [see Warnings and Precautions ( 5.8)].
Discontinuation
Advise patients not to stop taking Pristiq without talking first with their healthcare professional. Patients should be aware that discontinuation effects may occur when stopping Pristiq [see Warnings and Precautions ( 5.9) and Adverse Reactions ( 6.1)].
Interference with Cognitive and Motor Performance
Caution patients about operating hazardous machinery, including automobiles, until they are reasonably certain that Pristiq therapy does not adversely affect their ability to engage in such activities.
Alcohol
Advise patients to avoid alcohol while taking Pristiq [see Drug Interactions ( 7.5)].
Allergic Reactions
Advise patients to notify their physician if they develop allergic phenomena such as rash, hives, swelling, or difficulty breathing.
Pregnancy
Advise patients to notify their physician if they become pregnant or intend to become pregnant during therapy [see Use in Specific Populations ( 8.1)].
Nursing
Advise patients to notify their physician if they are breastfeeding an infant [see Use in Specific Populations (8.3)].
Residual Inert Matrix Tablet
Patients receiving Pristiq may notice an inert matrix tablet passing in the stool or via colostomy. Patients should be informed that the active medication has already been absorbed by the time the patient sees the inert matrix tablet.
Pristiq (desvenlafaxine) Full Prescribing Information
Detailed Info on Signs, Symptoms, Causes, Treatments of Depression
Wyeth Pharmaceuticals Inc.
Philadelphia, PA 19101
last updated: 04/08
APA Reference
Staff, H.
(2009, January 3). Pristiq (desvenlafaxine) Patient Information, HealthyPlace. Retrieved
on 2026, March 10 from https://www.healthyplace.com/other-info/psychiatric-medications/pristiq-patient-information