Caffeine Citrate: Stimulant (Full Prescribing Information)
Brand Name: Cafcit
Generic Name: Caffeine Citrate
Dosage Form: injection
Caffeine citrate is a central nervous system stimulant that is available as Cafcit, used to treat apnea in babies. Usage, dosage, side effects.
Description
Clinical Pharmacology
Indications and Usage
Warnings
Precautions
Adverse Reactions
Overdosage
Dosage and Administration
How Supplied
Caffeine citrate Patient Information (in plain English)
Description
Both Caffeine Citrate injection for intravenous administration and Caffeine Citrate oral solution are clear, colorless, sterile, non-pyrogenic, preservative-free, aqueous solutions adjusted to pH 4.7. Each mL contains 20 mg Caffeine Citrate (equivalent to 10 mg of caffeine base) prepared in solution by the addition of 10 mg caffeine anhydrous, USP to 5 mg citric acid monohydrate, USP, 8.3 mg sodium citrate dihydrate, USP and Water for Injection, USP.
Caffeine, a central nervous system stimulant, is an odorless white crystalline powder or granule, with a bitter taste. It is sparingly soluble in water and ethanol at room temperature. The chemical name of caffeine is 3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione. In the presence of citric acid it forms Caffeine Citrate salt in solution. The structural formula and molecular weight of Caffeine Citrate follows.
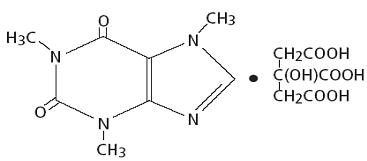
Caffeine Citrate
C14H18N4O9 Mol. Wt. 386.31
Clinical Pharmacology
Mechanism of Action
Caffeine is structurally related to other methylxanthines, theophylline, and theobromine. It is a bronchial smooth muscle relaxant, a CNS stimulant, a cardiac muscle stimulant, and a diuretic.
Although the mechanism of action of caffeine in apnea of prematurity is not known, several mechanisms have been hypothesized. These include: (1) stimulation of the respiratory center, (2) increased minute ventilation, (3) decreased threshold to hypercapnia, (4) increased response to hypercapnia, (5) increased skeletal muscle tone, (6) decreased diaphragmatic fatigue, (7) increased metabolic rate, and (8) increased oxygen consumption.
Most of these effects have been attributed to antagonism of adenosine receptors, both A1 and A2 subtypes, by caffeine, which has been demonstrated in receptor binding assays and observed at concentrations approximating those achieved therapeutically.
Pharmacokinetics
Absorption: After oral administration of 10 mg caffeine base/kg to preterm neonates, the peak plasma level (Cmax) for caffeine ranged from 6-10 mg/L and the mean time to reach peak concentration (Tmax) ranged from 30 minutes to 2 hours. The Tmax was not affected by formula feeding. The absolute bioavailability, however, was not fully examined in preterm neonates.
Distribution: Caffeine is rapidly distributed into the brain. Caffeine levels in the cerebrospinal fluid of preterm neonates approximate their plasma levels. The mean volume of distribution of caffeine in infants (0.8-0.9 L/kg) is slightly higher than that in adults (0.6 L/kg). Plasma protein binding data are not available for neonates or infants. In adults, the mean plasma protein binding in vitro is reported to be approximately 36%.
Metabolism: Hepatic cytochrome P450 1A2 (CYP1A2) is involved in caffeine biotransformation. Caffeine metabolism in preterm neonates is limited due to their immature hepatic enzyme systems.
Interconversion between caffeine and theophylline has been reported in preterm neonates; caffeine levels are approximately 25% of theophylline levels after theophylline administration and approximately 3-8% of caffeine administered would be expected to convert to theophylline.
Elimination: In young infants, the elimination of caffeine is much slower than that in adults due to immature hepatic and/or renal function. Mean half-life (T1/2) and fraction excreted unchanged in urine (Ae) of caffeine in infants have been shown to be inversely related to gestational/postconceptual age. In neonates, the T1/2 is approximately 3-4 days and the Ae is approximately 86% (within 6 days). By 9 months of age, the metabolism of caffeine approximates that seen in adults (T1/2 = 5 hours and Ae = 1%).
Special Populations: Studies examining the pharmacokinetics of caffeine in neonates with hepatic or renal insufficiency have not been conducted. Caffeine Citrate should be administered with caution in preterm neonates with impaired renal or hepatic function. Serum concentrations of caffeine should be monitored and dose administration of Caffeine Citrate should be adjusted to avoid toxicity in this population.
Clinical Studies
One multicenter, randomized, double-blind trial compared Caffeine Citrate to placebo in eighty-five (85) preterm infants (gestational age 28 to <33 weeks) with apnea of prematurity. Apnea of prematurity was defined as having at least 6 apnea episodes of greater than 20 seconds duration in a 24-hour period with no other identifiable cause of apnea. A 1 mL/kg (20 mg/kg Caffeine Citrate providing 10 mg/kg as caffeine base) loading dose of Caffeine Citrate was administered intravenously, followed by a 0.25 mL/kg (5 mg/kg Caffeine Citrate providing 2.5 mg/kg of caffeine base) daily maintenance dose administered either intravenously or orally (generally through a feeding tube). The duration of treatment in this study was limited to 10 to 12 days. The protocol allowed infants to be "rescued" with open-label Caffeine Citrate treatment if their apnea remained uncontrolled during the double-blind phase of the trial.
The percentage of patients without apnea on day 2 of treatment (24-48 hours after the loading dose) was significantly greater with Caffeine Citrate than placebo. The following table summarizes the clinically relevant endpoints evaluated in this study:clip
| Caffeine Citrate | Placebo | p-value | |
|---|---|---|---|
| Number of patients evaluated* | 45 | 37 | |
| % of patients with zero apnea events on day 2 | 26.7 | 8.1 | 0.03 |
| Apnea rate on day 2 (per 24 hrs.) | 4.9 | 7.2 | 0.134 |
| % of patients with 50% reduction in apnea events from baseline on day 2 | 76 | 57 | 0.07 |
| * Of 85 patients who received drug, 3 were not included in the efficacy analysis because they had <6 apnea episodes/24 hours at baseline. | |||
In this 10-12 day trial, the mean number of days with zero apnea events was 3 in the Caffeine Citrate group and 1.2 in the placebo group. The mean number of days with a 50% reduction from baseline in apnea events was 6.8 in the Caffeine Citrate group and 4.6 in the placebo group.
Indications and Usage
Caffeine Citrate injection and Caffeine Citrate oral solution are indicated for the short term treatment of apnea of prematurity in infants between 28 and <33 weeks gestational age.
Contraindications
Caffeine Citrate injection and Caffeine Citrate oral solution are contraindicated in patients who have demonstrated hypersensitivity to any of its components.
Warnings
During the double-blind, placebo-controlled clinical trial, 6 cases of necrotizing enterocolitis developed among the 85 infants studied (caffeine=46, placebo=39), with 3 cases resulting in death. Five of the six patients with necrotizing enterocolitis were randomized to or had been exposed to Caffeine Citrate.
Reports in the published literature have raised a question regarding the possible association between the use of methylxanthines and development of necrotizing enterocolitis, although a causal relationship between methylxanthine use and necrotizing enterocolitis has not been established. Therefore, as with all preterm infants, patients being treated with Caffeine Citrate should be carefully monitored for the development of necrotizing enterocolitis.
Precautions
General
Apnea of prematurity is a diagnosis of exclusion. Other causes of apnea (e.g., central nervous system disorders, primary lung disease, anemia, sepsis, metabolic disturbances, cardiovascular abnormalities, or obstructive apnea) should be ruled out or properly treated prior to initiation of Caffeine Citrate.
Caffeine is a central nervous system stimulant and in cases of caffeine overdose, seizures have been reported. Caffeine Citrate should be used with caution in infants with seizure disorders.
The duration of treatment of apnea of prematurity in the placebo-controlled trial was limited to 10 to 12 days. The safety and efficacy of Caffeine Citrate for longer periods of treatment have not been established. Safety and efficacy of Caffeine Citrate for use in the prophylactic treatment of sudden infant death syndrome (SIDS) or prior to extubation in mechanically ventilated infants have also not been established.
Cardiovascular
Although no cases of cardiac toxicity were reported in the placebo-controlled trial, caffeine has been shown to increase heart rate, left ventricular output, and stroke volume in published studies. Therefore, Caffeine Citrate should be used with caution in infants with cardiovascular disease.
Renal and Hepatic Systems
Caffeine Citrate should be administered with caution in infants with impaired renal or hepatic function. Serum concentrations of caffeine should be monitored and dose administration of Caffeine Citrate should be adjusted to avoid toxicity in this population. (See Clinical Pharmacology, Elimination, Special Populations.)
Information for Patients
Parents/caregivers of patients receiving Caffeine Citrate oral solution should receive the following instructions:
- Caffeine Citrate oral solution does not contain any preservatives and each vial is for single use only. Any unused portion of the medication should be discarded.
- It is important that the dose of Caffeine Citrate oral solution be measured accurately, i.e., with a 1cc or other appropriate syringe.
- Consult your physician if the baby continues to have apnea events; do not increase the dose of Caffeine Citrate oral solution without medical consultation.
- Consult your physician if the baby begins to demonstrate signs of gastrointestinal intolerance, such as abdominal distention, vomiting, or bloody stools, or seems lethargic.
- Caffeine Citrate oral solution should be inspected visually for particulate matter and discoloration prior to its administration. Vials containing discolored solution or visible particulate matter should be discarded.
Laboratory Tests
Prior to initiation of Caffeine Citrate, baseline serum levels of caffeine should be measured in infants previously treated with theophylline, since preterm infants metabolize theophylline to caffeine. Likewise, baseline serum levels of caffeine should be measured in infants born to mothers who consumed caffeine prior to delivery, since caffeine readily crosses the placenta.
In the placebo-controlled clinical trial, caffeine levels ranged from 8 to 40 mg/L. A therapeutic plasma concentration range of caffeine could not be determined from the placebo-controlled clinical trial. Serious toxicity has been reported in the literature when serum caffeine levels exceed 50 mg/L. Serum concentrations of caffeine may need to be monitored periodically throughout treatment to avoid toxicity.
In clinical studies reported in the literature, cases of hypoglycemia and hyperglycemia have been observed. Therefore, serum glucose may need to be periodically monitored in infants receiving Caffeine Citrate.
Drug Interactions
Cytochrome P450 1A2 (CYP1A2) is known to be the major enzyme involved in the metabolism of caffeine. Therefore, caffeine has the potential to interact with drugs that are substrates for CYP1A2, inhibit CYP1A2, or induce CYP1A2.
Few data exist on drug interactions with caffeine in preterm neonates. Based on adult data, lower doses of caffeine may be needed following coadministration of drugs which are reported to decrease caffeine elimination (e.g., cimetidine and ketoconazole) and higher caffeine doses may be needed following coadministration of drugs that increase caffeine elimination (e.g., phenobarbital and phenytoin).
Caffeine administered concurrently with ketoprofen reduced the urine volume in four healthy volunteers. The clinical significance of this interaction in preterm neonates is not known.
Interconversion between caffeine and theophylline has been reported in preterm neonates. The concurrent use of these drugs is not recommended.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year study in Sprague-Dawley rats, caffeine (as caffeine base) administered in drinking water was not carcinogenic in male rats at doses up to 102 mg/kg or in female rats at doses up to 170 mg/kg (approximately 2 and 4 times, respectively, the maximum recommended intravenous loading dose for infants on a mg/m2 basis). In an 18-month study in C57BL/6 mice, no evidence of tumorigenicity was seen at dietary doses up to 55 mg/kg (less than the maximum recommended intravenous loading dose for infants on a mg/m2 basis).
Caffeine (as caffeine base) increased the sister chromatid exchange (SCE) SCE/cell metaphase (exposure time dependent) in an in vivo mouse metaphase analysis. Caffeine also potentiated the genotoxicity of known mutagens and enhanced the micronuclei formation (5-fold) in folate-deficient mice. However, caffeine did not increase chromosomal aberrations in in vitro Chinese hamster ovary cell (CHO) and human lymphocyte assays and was not mutagenic in an in vitro CHO/hypoxanthine guanine phosphoribosyltransferase (HGPRT) gene mutation assay, except at cytotoxic concentrations. In addition, caffeine was not clastogenic in an in vivo mouse micronucleus assay.
Caffeine (as caffeine base) administered to male rats at 50 mg/kg/day subcutaneously (approximately equal to the maximum recommended intravenous loading dose for infants on a mg/m2 basis) for 4 days prior to mating with untreated females, caused decreased male reproductive performance in addition to causing embryotoxicity. In addition, long-term exposure to high oral doses of caffeine (3 g over 7 weeks) was toxic to rat testes as manifested by spermatogenic cell degeneration.
Pregnancy: Pregnancy Category C
Concern for the teratogenicity of caffeine is not relevant when administered to infants. In studies performed in adult animals, caffeine (as caffeine base) administered to pregnant mice as sustained release pellets at 50 mg/kg (less than the maximum recommended intravenous loading dose for infants on a mg/m2 basis), during the period of organogenesis, caused a low incidence of cleft palate and exencephaly in the fetuses. There are no adequate and well-controlled studies in pregnant women.
Adverse Reactions
Overall, the reported number of adverse events in the double-blind period of the controlled trial was similar for the Caffeine Citrate and placebo groups. The following table shows adverse events that occurred in the double-blind period of the controlled trial and that were more frequent in Caffeine Citrate treated patients than placebo.
| Adverse Event (AE) | Caffeine Citrate N=46 n (%) |
Placebo N=39 n (%) |
|---|---|---|
| BODY AS A WHOLE | ||
| Accidental Injury | 1 (2.2) | 0 (0.0) |
| Feeding Intolerance | 4 (8.7) | 2 (5.1) |
| Sepsis | 2 (4.3) | 0 (0.0) |
| CARDIOVASCULAR SYSTEM | ||
| Hemorrhage | 1 (2.2) | 0 (0.0) |
| DIGESTIVE SYSTEM | ||
| Necrotizing Enterocolitis | 2 (4.3) | 1 (2.6) |
| Gastritis | 1 (2.2) | 0 (0.0) |
| Gastrointestinal Hemorrhage | 1 (2.2) | 0 (0.0) |
| HEMIC AND LYMPHATIC SYSTEM | ||
| Disseminated Intravascular Coagulation | 1 (2.2) | 0 (0.0) |
| METABOLIC AND NUTRITIVE DISORDERS | ||
| Acidosis | 1 (2.2) | 0 (0.0) |
| Healing Abnormal | 1 (2.2) | 0 (0.0) |
| NERVOUS SYSTEM | ||
| Cerebral Hemorrhage | 1 (2.2) | 0 (0.0) |
| RESPIRATORY SYSTEM | ||
| Dyspnea | 1 (2.2) | 0 (0.0) |
| Lung Edema | 1 (2.2) | 0 (0.0) |
| SKIN AND APPENDAGES | ||
| Dry Skin | 1 (2.2) | 0 (0.0) |
| Rash | 4 (8.7) | 3 (7.7) |
| Skin Breakdown | 1 (2.2) | 0 (0.0) |
| SPECIAL SENSES | ||
| Retinopathy of Prematurity | 1 (2.2) | 0 (0.0) |
| UROGENITAL SYSTEM | ||
| Kidney Failure | 1 (2.2) | 0 (0.0) |
In addition to the cases above, three cases of necrotizing enterocolitis were diagnosed in patients receiving Caffeine Citrate during the open-label phase of the study.
Three of the infants who developed necrotizing enterocolitis during the trial died. All had been exposed to caffeine. Two were randomized to caffeine, and one placebo patient was "rescued" with open-label caffeine for uncontrolled apnea.
Adverse events described in the published literature include: central nervous system stimulation (i.e., irritability, restlessness, jitteriness), cardiovascular effects (i.e., tachycardia, increased left ventricular output, and increased stroke volume), gastrointestinal effects (i.e., increased gastric aspirate, gastrointestinal intolerance), alterations in serum glucose (hypoglycemia and hyperglycemia) and renal effects (increased urine flow rate, increased creatinine clearance, and increased sodium and calcium excretion). Published long-term follow-up studies have not shown caffeine to adversely affect neurological development or growth parameters.
Overdosage
Following overdose, serum caffeine levels have ranged from approximately 24 mg/L (a post marketing spontaneous case report in which an infant exhibited irritability, poor feeding and insomnia) to 350 mg/L. Serious toxicity has been associated with serum levels greater than 50 mg/L (see Precautions-Laboratory Tests and Dosage and Administration). Signs and symptoms reported in the literature after caffeine overdose in preterm infants include fever, tachypnea, jitteriness, insomnia, fine tremor of the extremities, hypertonia, opisthotonos, tonic-clonic movements, nonpurposeful jaw and lip movements, vomiting, hyperglycemia, elevated blood urea nitrogen, and elevated total leukocyte concentration. Seizures have also been reported in cases of overdose. One case of caffeine overdose complicated by development of intraventricular hemorrhage and long-term neurological sequelae has been reported. Another case of Caffeine Citrate overdose (from New Zealand) of an estimated 600 mg Caffeine Citrate (approximately 322 mg/kg) administered over 40 minutes was complicated by tachycardia, ST depression, respiratory distress, heart failure, gastric distention, acidosis and a severe extravasation burn with tissue necrosis at the peripheral intravenous injection site. No deaths associated with caffeine overdose have been reported in preterm infants.
Treatment of caffeine overdose is primarily symptomatic and supportive. Caffeine levels have been shown to decrease after exchange transfusions. Convulsions may be treated with intravenous administration of diazepam or a barbiturate such as pentobarbital sodium.
Dosage and Administration
Prior to initiation of Caffeine Citrate, baseline serum levels of caffeine should be measured in infants previously treated with theophylline, since preterm infants metabolize theophylline to caffeine. Likewise, baseline serum levels of caffeine should be measured in infants born to mothers who consumed caffeine prior to delivery, since caffeine readily crosses the placenta.
The recommended loading dose and maintenance doses of Caffeine Citrate follow.
| Dose of Caffeine Citrate Volume |
Dose of Caffeine Citrate mg/kg |
Route | Frequency | |
|---|---|---|---|---|
| Loading Dose | 1 mL/kg | 20 mg/kg | Intravenous* (over 30 minutes) | One Time |
| Maintenance Dose | 0.25 mL/kg | 5 mg/kg | Intravenous* (over 10 minutes) or Orally | Every 24 hours** |
| * using a syringe infusion pump ** beginning 24 hours after the loading dose |
||||
NOTE THAT THE DOSE OF CAFFEINE BASE IS ONE-HALF THE DOSE WHEN EXPRESSED AS Caffeine Citrate (e.g., 20 mg of Caffeine Citrate is equivalent to 10 mg of caffeine base).
Serum concentrations of caffeine may need to be monitored periodically throughout treatment to avoid toxicity. Serious toxicity has been associated with serum levels greater than 50 mg/L.
Caffeine Citrate injection and Caffeine Citrate oral solution should be inspected visually for particulate matter and discoloration prior to administration. Vials containing discolored solution or visible particulate matter should be discarded.
Drug Compatibility
To test for drug compatibility with common intravenous solutions or medications, 20 mL of Caffeine Citrate injection were combined with 20 mL of a solution or medication, with the exception of an Intralipid® admixture, which was combined as 80 mL/80 mL. The physical appearance of the combined solutions was evaluated for precipitation. The admixtures were mixed for 10 minutes and then assayed for caffeine. The admixtures were then continually mixed for 24 hours, with further sampling for caffeine assays at 2, 4, 8, and 24 hours.
Based on this testing, Caffeine Citrate injection, 60 mg/3 mL is chemically stable for 24 hours at room temperature when combined with the following test products.
- Dextrose Injection, USP 5%
- 50% Dextrose Injection USP
- Intralipid® 20% IV Fat Emulsion
- Aminosyn® 8.5% Crystalline Amino Acid Solution
- Dopamine HCI Injection, USP 40 mg/mL diluted to 0.6 mg/mL with Dextrose Injection, USP 5%
- Calcium Gluconate Injection, USP 10% (0.465 mEq/Ca+2/mL)
- Heparin Sodium Injection, USP 1000 units/mL diluted to 1 unit/mL with Dextrose Injection, USP 5%
- Fentanyl Citrate Injection, USP 50 µg/mL diluted to 10 µg/mL with Dextrose Injection, USP 5%
How Supplied
Both Caffeine Citrate injection and Caffeine Citrate oral solution are available as clear, colorless, sterile, non-pyrogenic, preservative-free, aqueous solutions in 5 mL colorless glass vials. The vials of Caffeine Citrate injection are sealed with gray rubber stopper and white flip off aluminum seal printed with "FOR INTRAVENOUS USE ONLY" in red. The vials of Caffeine Citrate oral solution are sealed with gray rubber stopper and a dark blue matte finish, flip off tear off aluminum seal printed with "FOR ORAL USE ONLY - FLIP UP & TEAR" in white.
Both the injection and oral solution vials contain 3 mL solution at a concentration of 20 mg/mL Caffeine Citrate (60 mg/vial) equivalent to 10 mg/mL caffeine base (30 mg/vial).
Caffeine Citrate injection, USP
NDC 47335-289-40: 3 mL vial, individually packaged in a carton.
Caffeine Citrate oral solution, USP
NDC 47335-290-44: 3 mL vial (NOT CHILD-RESISTANT), 10 vials per white
polypropylene child-resistant container.
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° and 30°C (59° and 86°F) [see USP Controlled Room Temperature].
Preservative free. For single use only. Discard unused portion.
ATTENTION PHARMACIST: Detach "Instructions for Use" from the package insert and dispense with Caffeine Citrate oral solution prescription.
Distributed by:
Caraco Pharmaceutical Laboratories, Ltd.
1150 Elijah McCoy Drive, Detroit, MI 48202
Manufactured by:
Sun Pharmaceutical Ind. Ltd.
Halol-Baroda Highway,
Halol-389 350, Gujarat, India.
last updated 02/2010
Caffeine citrate Patient Information (in plain English)
Detailed Info on Signs, Symptoms, Causes, Treatments of Sleep Disorders
The information in this monograph is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects. This information is generalized and is not intended as specific medical advice. If you have questions about the medicines you are taking or would like more information, check with your doctor, pharmacist, or nurse.
back to:
~ all articles on sleeping disorders
APA Reference
Staff, H.
(2019, August 28). Caffeine Citrate: Stimulant (Full Prescribing Information), HealthyPlace. Retrieved
on 2026, March 3 from https://www.healthyplace.com/other-info/sleep-disorders/caffeine-citrate-stimulant-full-prescribing-information
